Water Pollution
Water is everywhere and a lot of it is polluted, particularly near population centers.
Pollution
Pollutant can mean a few things:
- A chemical out of place
- A substance out of place
- A substance in high concentration that causes a nuisance or hazard
- Physical pollution: e.g., suspended sediment
- Thermal pollution: e.g., excess heat
- Chemical pollution is of most concern
Geochemical Cycles
Geochemistry
Interaction of geology and chemistry
- Rocks experience the rock cycle
- Water experiences the hydrologic cycle
- Environmental chemicals experience different and specific geochemical cycles
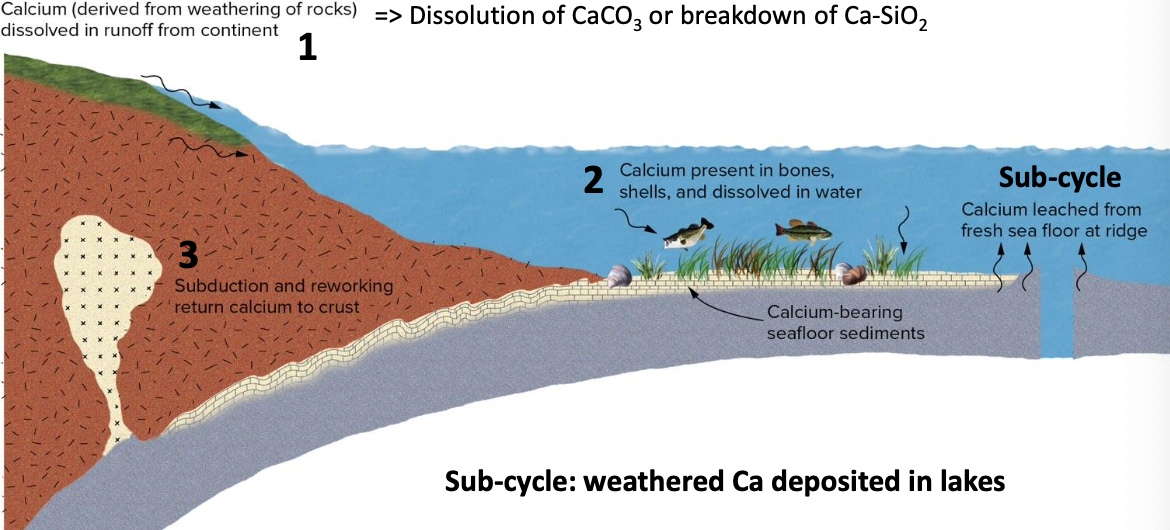
- Ca is weathered from rocks and transported to ocean
- CA is taken up by ocean organisms and later deposited and lithified
- Ca-sediments can be matamorphosed, subducted, or appear as continental crust
Residence Time
Residence Time
Measure of how rapidly a chemical cycles through a reservoir
- E.g., reservoirs: mantle, continental crust, marine deposits, dissolved in ocean

- Residence Time: how long the substance stays in the reservoir
- Capacity – maximum concentration of the substance a reservoir can reach before saturation occurs
- Saturation is the level at which a reservoir can hold no more of a substance
- Rate of Input – how much of the substance is being brought into the reservoir
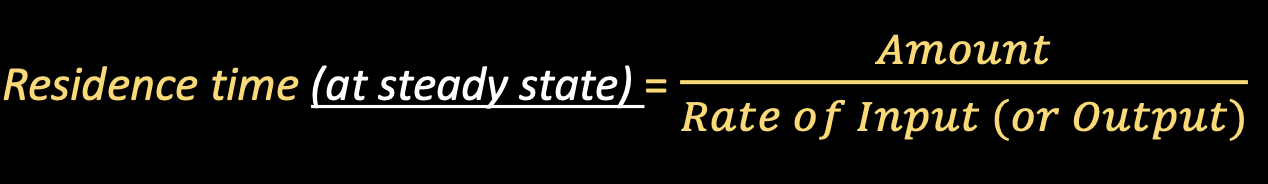
- Amount – current amount of substance in the reservoir
- Rate of input or output – how much is coming in or going out
- Rate of input = rate of output when at steady state
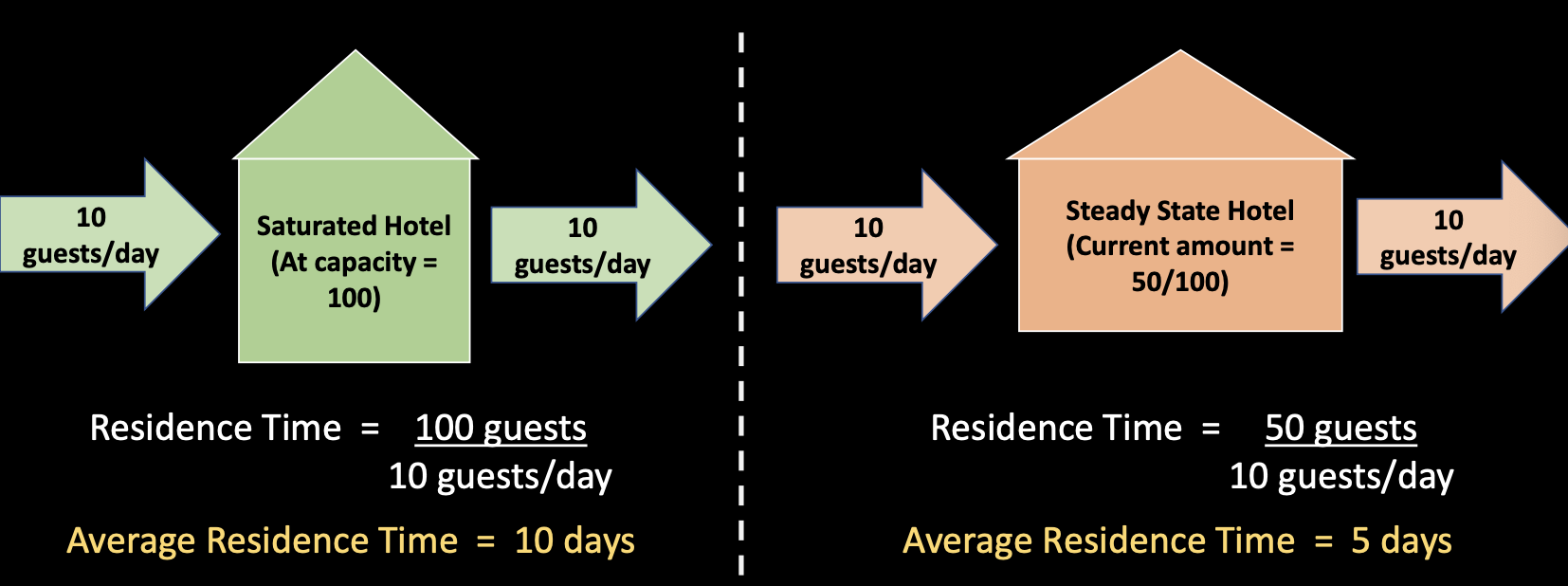
Both hotels are in steady state, just one is at capacity. This calculates an average residence time, some guests stay longer, some shorter.
Residence Time & Pollution
- Residence time (RT) of natural chemicals are mostly known, but not of many synthetic chemicals
- Residence time
- Tells you how long that pollutant will be a problem
- is dependent on other processes impacting the pollutant
Trace Elements, Health, and Pollution
Trace Elements
Elements that occur in very low concentrations in rocks, soil, plants, animals, or humans
- Lack of trace elements can be harmful or beneficial (e.g., iodine)
- Some trace elements are harmful or beneficial at low doses and harmful at higher ones (e.g., fluorine)
- Look at slides for examples
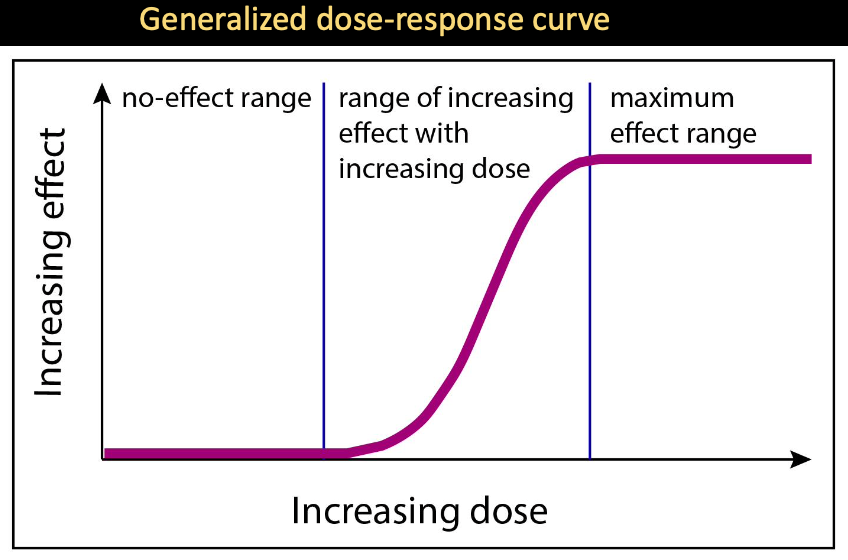
Dose-response curves
show the relative benefit/harm of an element based on dosage
- Not possible to get for every chemical because it requires experimentation on human
- And isolating the chemical is complicated because of lots of processes
Point and Nonpoint Pollution Sources
Point Sources: pollutant released from one identifiable source
- E.g., sewer outlet; factory outlet; septic tank
- Easier to identify and to monitor, thus controlled more than nonpoint sources
Nonpoint Sources: Diffuse sources coming from a wider area and potentially multiple entry points
- E.g, fertilizer/pesticide runoff from farms; acid drainage from strip mine; road salt runoff from street
Types of Sources Pollution
- Organic Matter
- Agricultural Pollution
- Industrial Pollution
- Groundwater Pollution
Organic Matter
What is Organic Matter and where does it come from?
- Organic matter: living and dead organisms and their by-products
- Sources:
- Runoff form an animal feedlot (major source)
- Discharge from food processing plants
- Human or animal waste
- Algae in a pond
- Runoff from municipal streets or highways
- Organic matter is broken down by microbes
- If ample oxygen is available, then aerobic decomposition occurs depletion
- If oxygen is depleted, then anaerobic decomposition occurs and release
Organic matter depletes oxygen!
- Biological oxygen demand (BOD): amount of oxygen required to breakdown the organic matter aerobically
- More organic matter, higher the BOD
- Oxygen sag curve: dissolved oxygen (DO) as function of distance from waste source
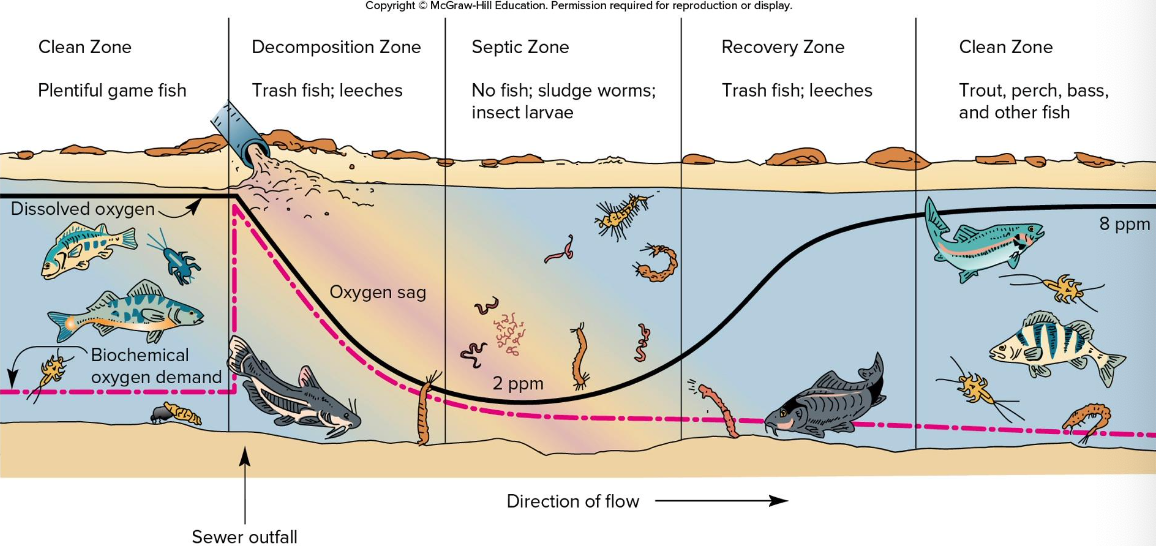
- Start with saturated oxygen levels and clean water full of fish, then a pollutant causes a high BOD and depletes for awhile, which changes the ecosystem
- Eventually with distance the pollutant is depleted, and the water recovers to saturated levels and lots of fish
- Removal of organic matter to reduce BOD is key function of sewage treatment plants
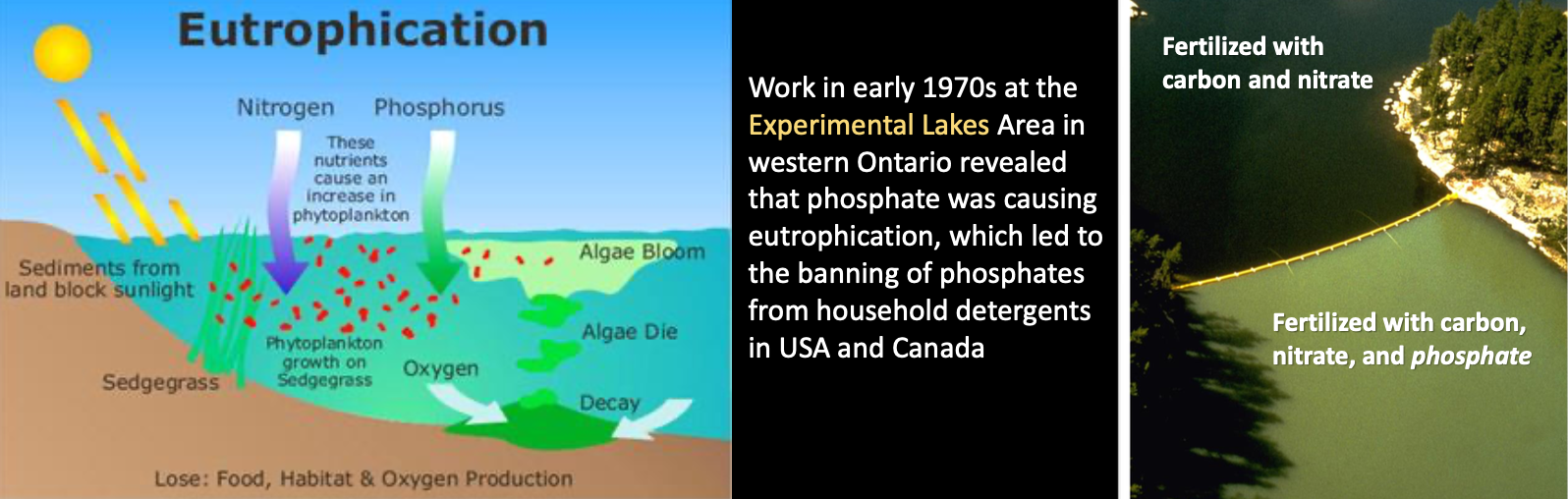
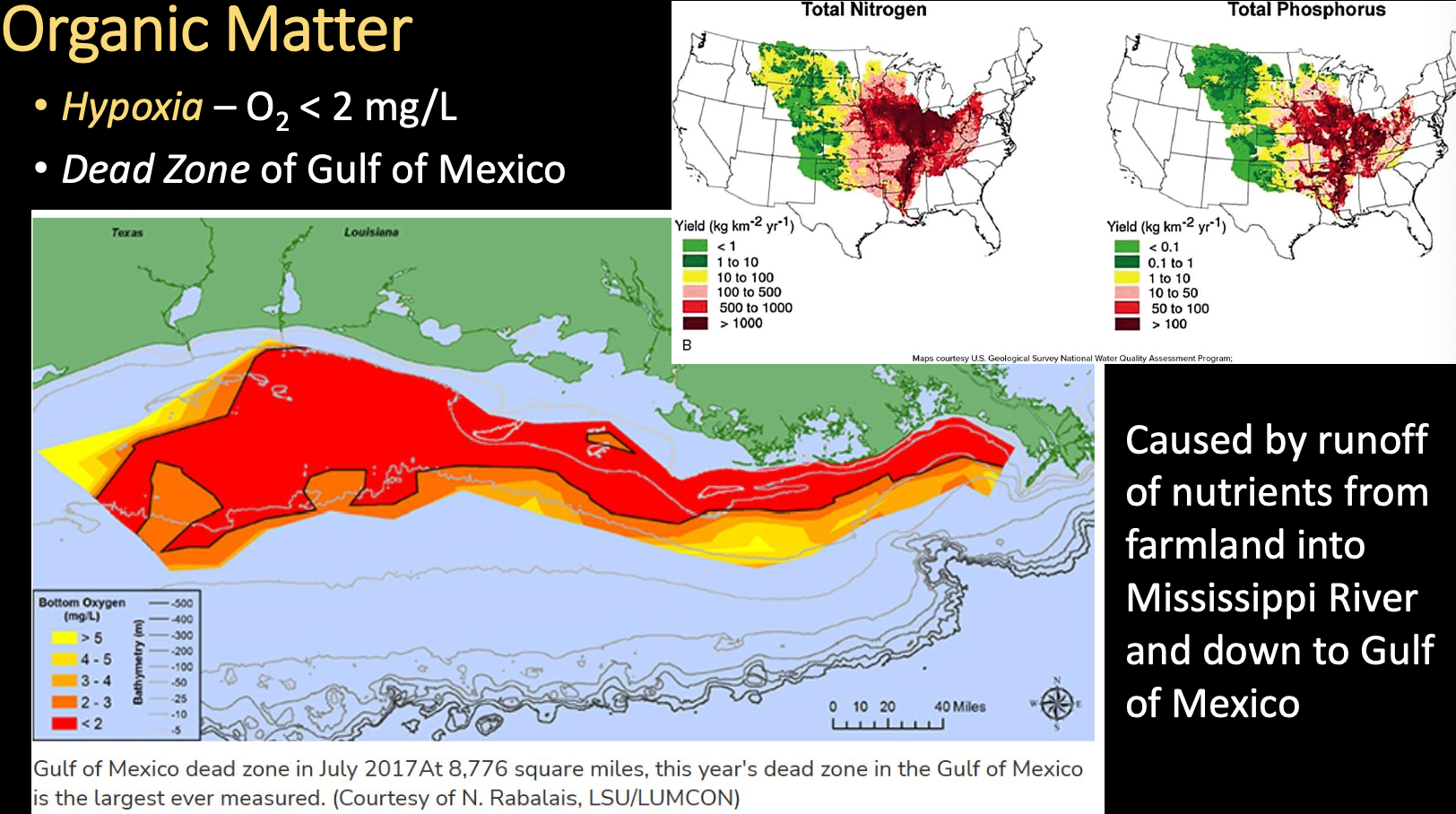
Eutrophication
Results of excess nutrients (nitrates, phosphates, sulfates) in the water from the breakdown of organic matter or direct sources like fertilizer runoff from farms (i.e., a body of water is or has become eutrophic)
- Enhances algal growth, increase BOD and anoxia, but can be natural process in a water body
Agricultural Pollution
- Pesticides and fertilizers found in surface water, groundwater, fish and sediments
- In both farm and urban lands
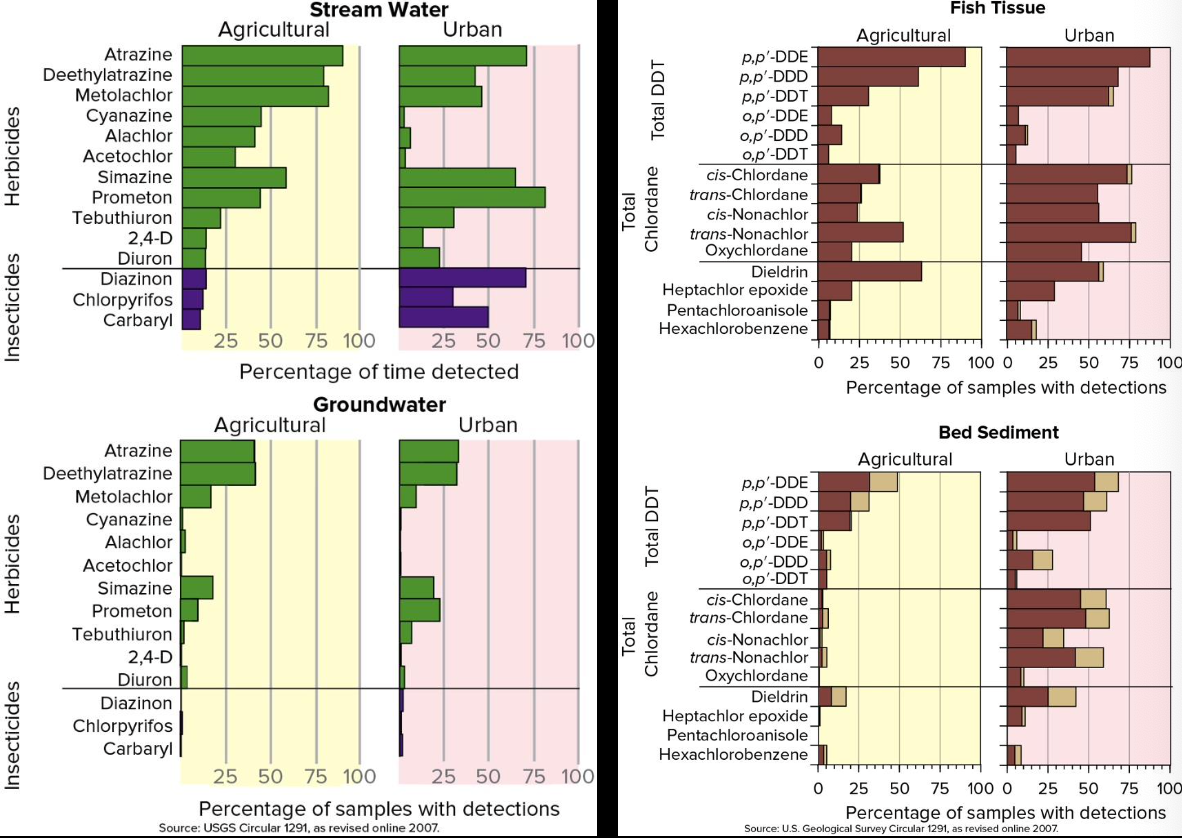
Pesticides
- Chemicals in the pesticide, chemicals used to make the pesticide (e.g., dioxin from the Love Canal story), and by products of reactions can all be toxic
Mitigation Strategies:
- Reduce spring application to reduce runoff spikes
- Apply when necessary, instead of the whole growing season
- May help hinder development of resistance by the pests
- Nonchemical strategies using other organisms
- GMO crops
Fertilizers consist of nitrates, phosphates, and potash
- Soluble and not immediately taken up by plants runoff and eutrophication
- Mitigation: reduce use, slow-release fertilizer, legumes with nitrogen-fixing bacteria on their roots
- Can we use manure instead?
Manure consists of organic matter and nutrients
- Higher BOD and eutrophication
- Feedlots produce a lot of waste that can be subject to erosion, particularly by water
Mitigation: diversion into basins and produce fertilizer; slopes down to planted filter basins effective but pollutes groundwater
Sediment erosion is natural, but agriculture increases erosion 4-9x
- Sediment pollution in agricultural area is a serious water-quality issue
Issues:
- Unsuitable for swimming or drinking
- Reduces light
- Fills in channels and reservoirs
- Clogs water filters
- Damages power-generating equipment
Industrial Pollution
We do NOT know the toxicity of most chemicals created each year.
- National Research Council study found 70% of 66,000 chemicals had no visible toxicity data
- In 1990, the ten millionth new chemical was registered by ACS
- Inorganic pollutants, starting with metals
- Organic pollutants ( majority of newly created)
- Thermal pollution
Inorganic Pollutants - Metals
- The paper, petroleum, chemical and primary metals industries can release
- Arsenic, cadmium, chromium, copper, lead, mercury, nickel, uranium, and zinc
- Arsenic can cause cancer or death when ingested at high levels
- Some regions naturally have it in groundwater (e.g., Bangladesh)
- Some metals bioaccumulate in organisms by moving up the food chain (e.g., mercury, lead, cadmium and plutonium) → biomagnification
- Mercury in fish we eat acts on nervous system and brain
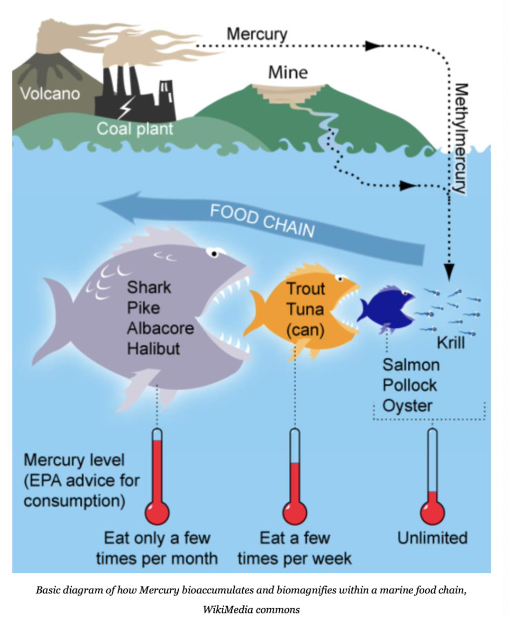
Other Inorganic Pollutants
- Chlorine –
- used to kill bacteria in water- and sewage-treatment plants also toxic to algae and fish
- Acid mine drainage
- from coal and sulfide mines pollutes as well as leaches toxic metals from tailings
- Asbestos mine waste
- was dumped in surface waters, including the Great Lakes where people were drinking it and effects still unknown
Organic Pollutants
- Most newly created chemicals are organic
- Pesticides, e.g., dioxin and DDT
- Oil spills
- PCBs (polychlorinated biphenyls) for insulating fluid in electrical equipmentvand as plasticizers
- MTBE (methyl tertbutyl ether), added to gasoline, is in groundwater but don’t know the effects of it yet
- Pharmaceuticals – we know very little about, but they are in our water
Mitigation or Controlling:
- Treating wastewater is expensive and becomes more costly as you near ‘pure water’
- Ultimately you may just displace the pollutants and now deal with a toxic waste removal situation
- Some have really long residence times no matter what you try
Thermal Pollution
Electricity-generating plants and others that use cooling water heat local waters
- Extreme temperatures may destroy populations by disrupting breeding
- Changes rates of chemical reactions and microbial activity
- Decreases solubility, so less gas dissolves Mitigation: Holding waters to cool may help
- But overall heat is not long-lasting
Groundwater Pollution
Groundwater can be polluted by point and nonpoint sources
- Reflects what is happening above
- Lag time between introduction of pollutants and time of appearance
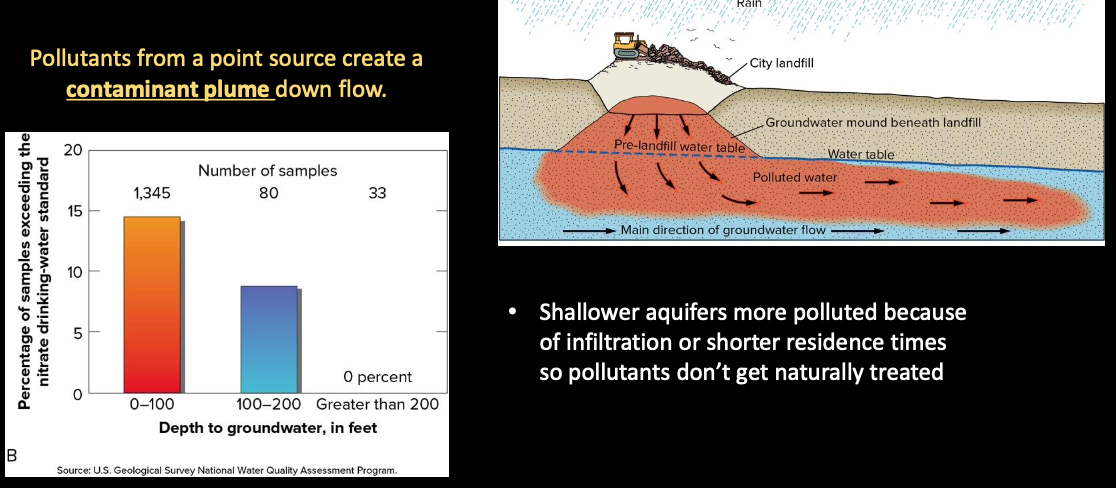
Reversing the Damage to Groundwater
Very difficult to mitigate or resolve groundwater pollution
- Need extensive well network to monitor
- Can spread before you realize it is there
- May reappear intermittently because stored somewhere or interacts
- In situ treatments not as effective because of limited mixing and slow migration
- Treatment after extraction may be possible
Only approach is sometimes waiting for natural processes to remove it
Decontamination after extraction = pump-and-treat method
Treatments include:
- Adjusting acidity (pH) or adding substance to precipitate out pollutant ⇒ sludge
- Use microbial organisms to break down organic matter like in sewage treatment
- Air stripping removes volatile organics from water into air
- Activated charcoal/carbon adsorbs organics
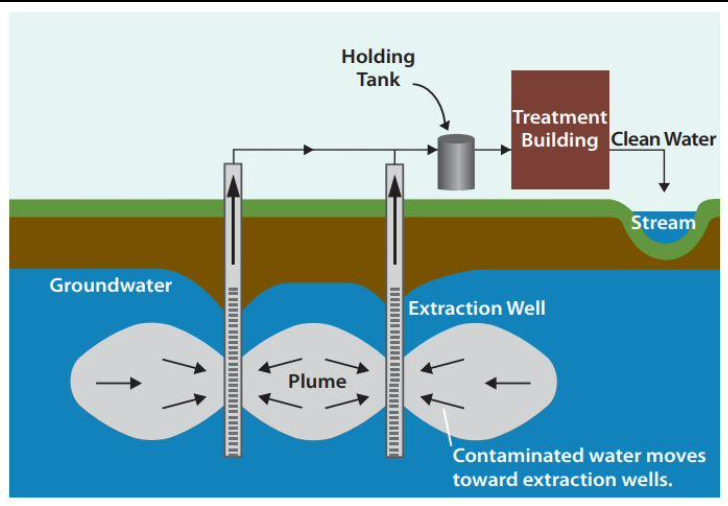
In situ decontamination is only possible when type and extent of pollution is defined, and is site- and pollutant-specific
Treatments include:
- Immobilization via precipitation for inorganic, e.g., metals
- Bioremediation (breakdown by microbes) for organics
- For petroleum products that float on top of water, pump it out
- Permeable reactive barrier – material to remove pollutant in flow path
- Petroleum adsorbs to carbon particles
- Metals precipitate, e.g., lead with limestone
- Reactions leave less toxic, e.g., iron with solvent
- Microbial breakdown or conversion
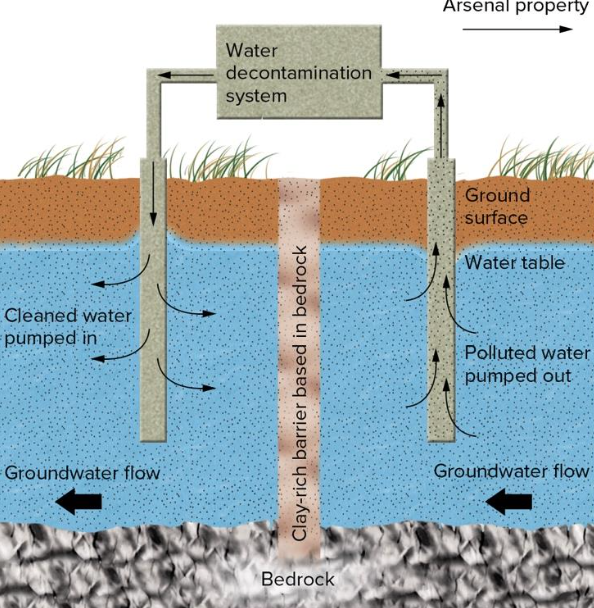
Containment: Isolate problem locally
- Rocky Mountain Arsenal, Colorado, USA
- Careless toxic waste disposal polluted groundwater and caused crop damage
- Physical barrier of clay-rich material (impermeable) to prevent flow and allow pump-and-treat remediation