Air Pollution
Atmospheric Chemistry
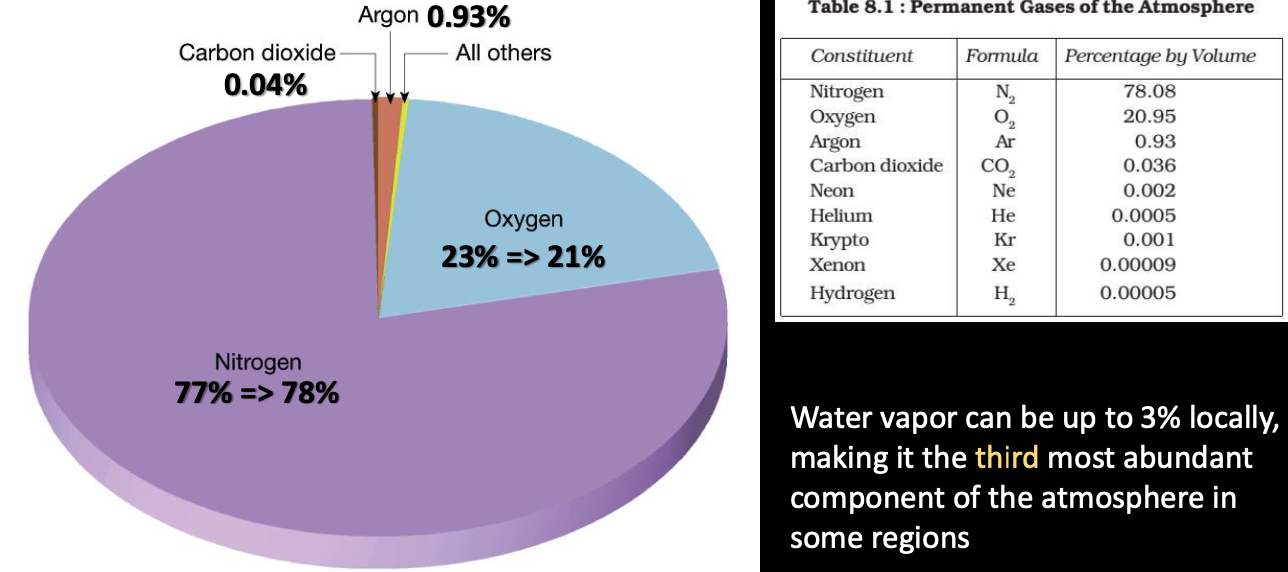
Atmospheric gases have residence times in the atmosphere (and any other reservoirs they may exist) influenced by amount present and rates of addition and removal of them:
- 7 million years
- 100-200 years
- Argon and other trace gases, helium and neon ⇒ infinite because so inert
- unknown because N-cycle is very complex
- But we do know the residence times for some N-compounds
- (dinitrogen, mostly in our atmosphere) ⇒ 44 million years
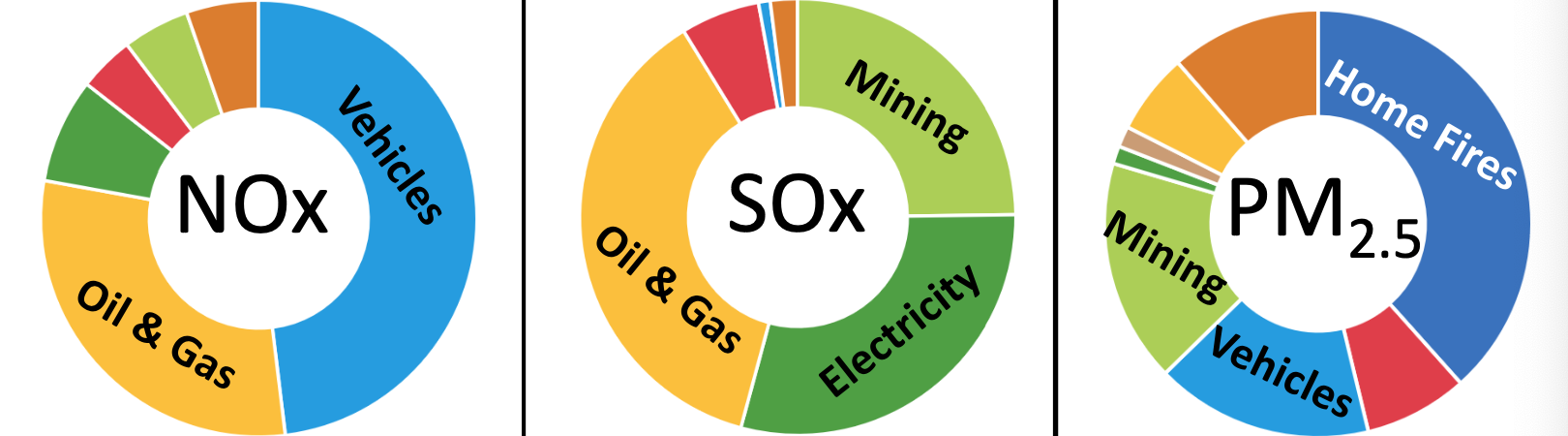
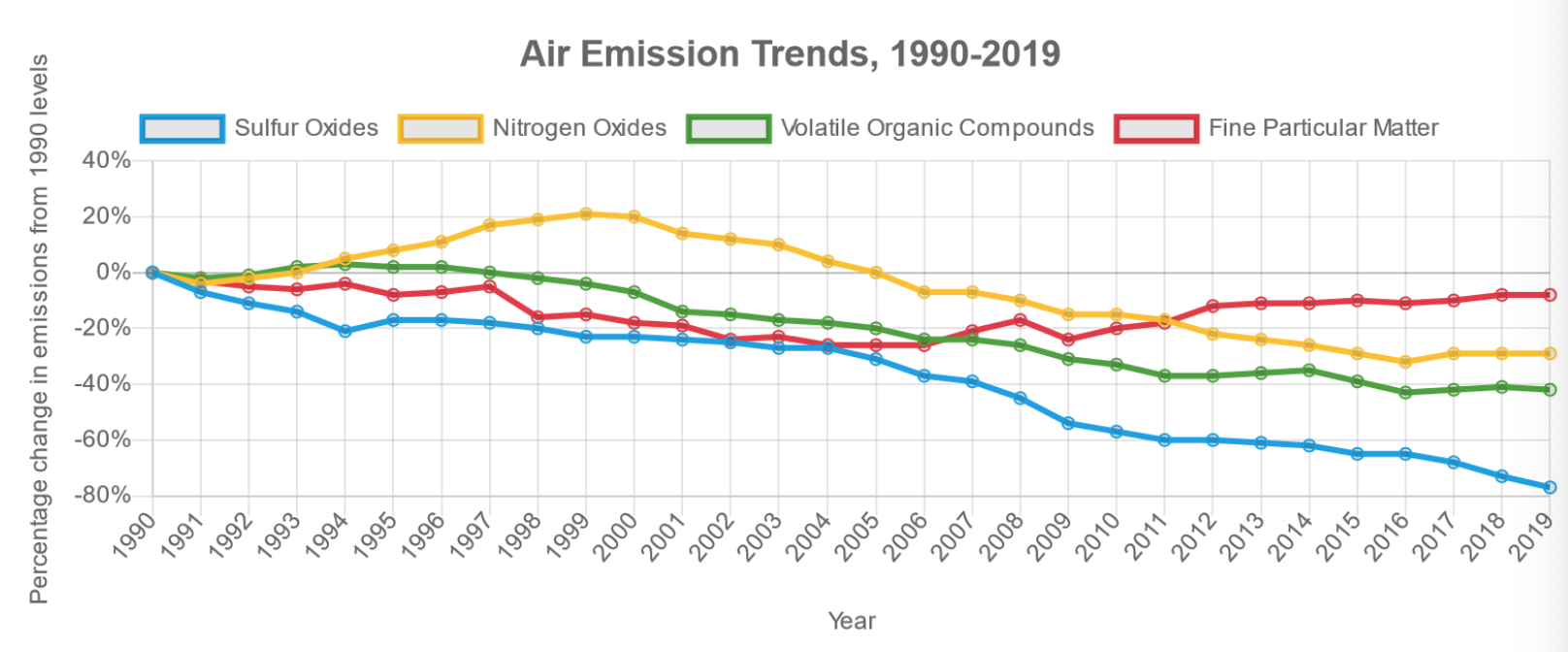
Types and Sources of Air Pollution
- Particulates
- Carbon gases
- Sulfur gases
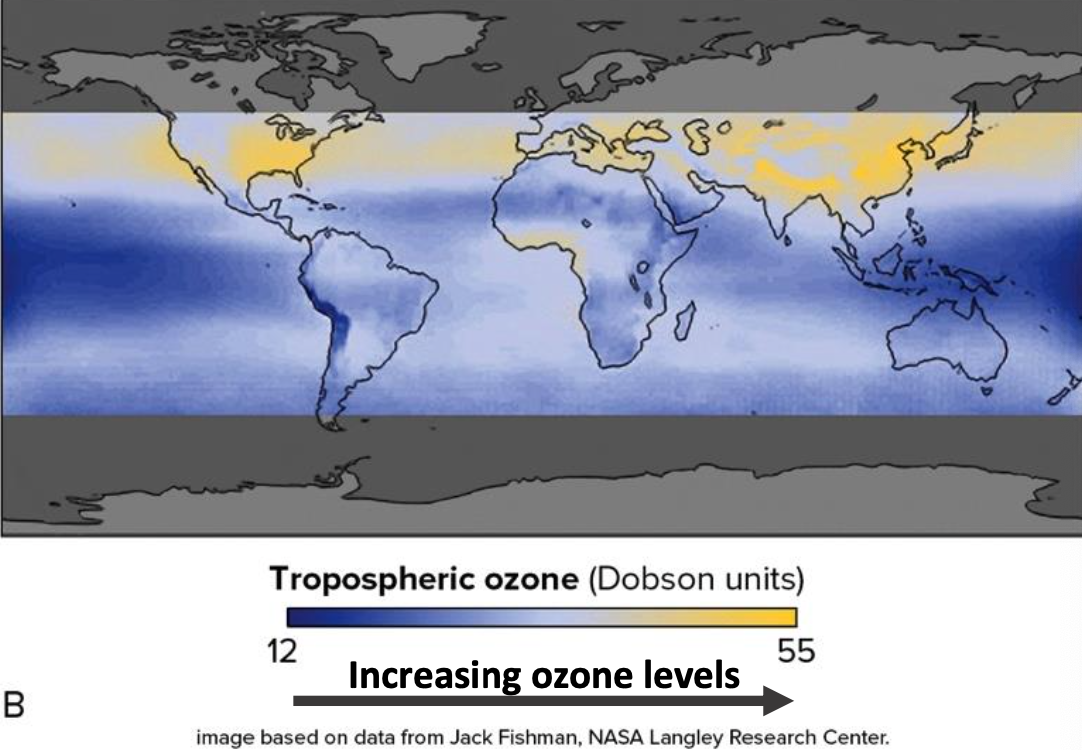
- Nitrogen gases and ‘Smog Ozone’
- The ozone layer and CFCs
- Lead
- Others
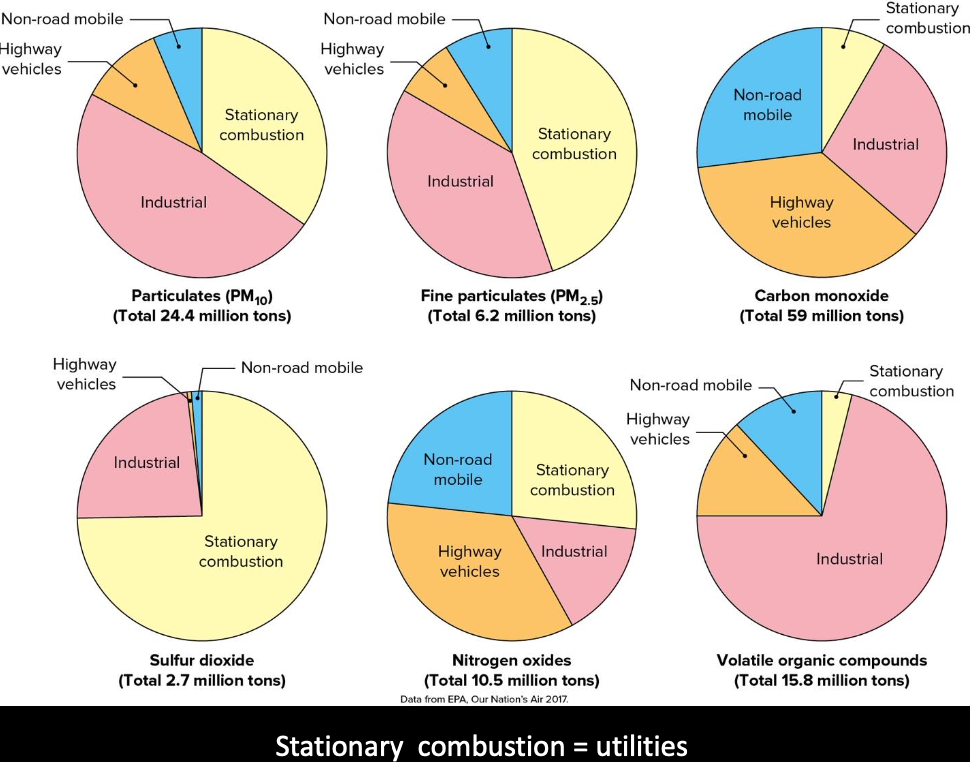
Particulates
Mainly generated by humans from point sources:
- Soot, smoke, ash from fuel (coal) combustion
- Dust from industrial processing
- Other solids from burning vegetation
- Estimates vary from 35 – 180 million tons/year
Natural sources of particulates include:
- Volcanic eruptions
- Forest fires
- Wind erosion of dust
- Salt spray off the ocean
Short residence times:
- Removed primarily by precipitation
- Mostly a local problem
Problems from particulate pollution include
- Ash fouling equipment
- Fine rock and mineral dust being carcinogenic, toxic, or simply unhealthy
- Coal ash containing heavy metals and uranium
- Inhalation of fine particles emitted or created in atmosphere
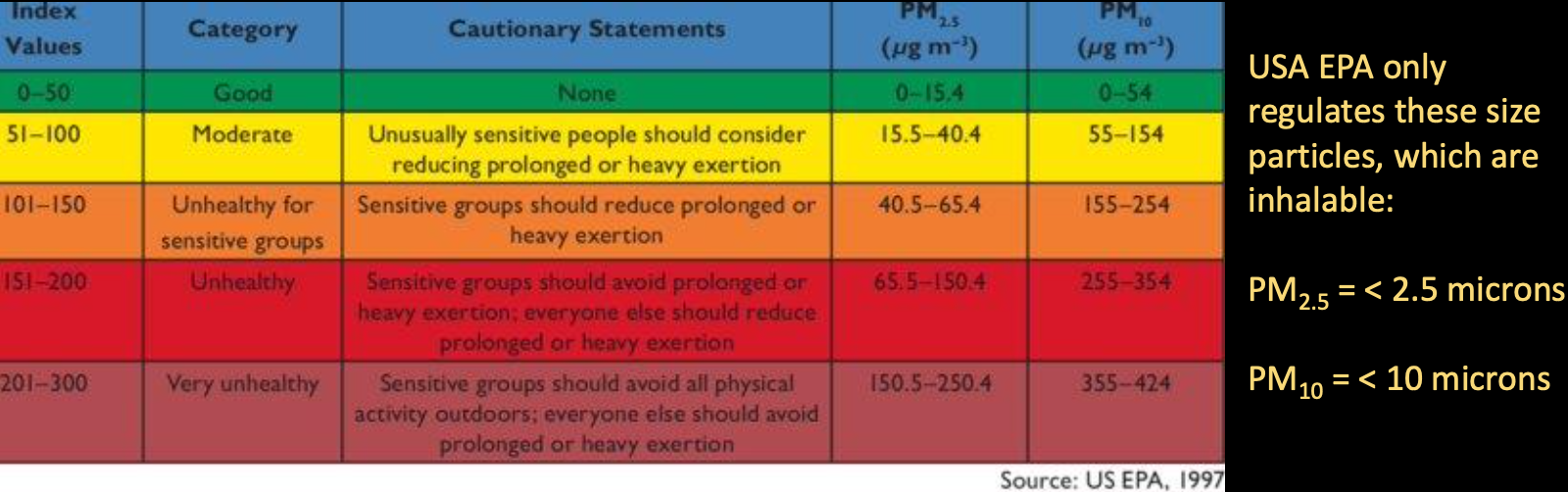
- 80% of global population lives with , (WHO’s maximum exposure limit)
- China burns a lot of coal
- Africa burns a lot of vegetation
Long term exposure to PM2.5 particulates may be responsible for >30 million premature deaths in China between 2000 and 2016
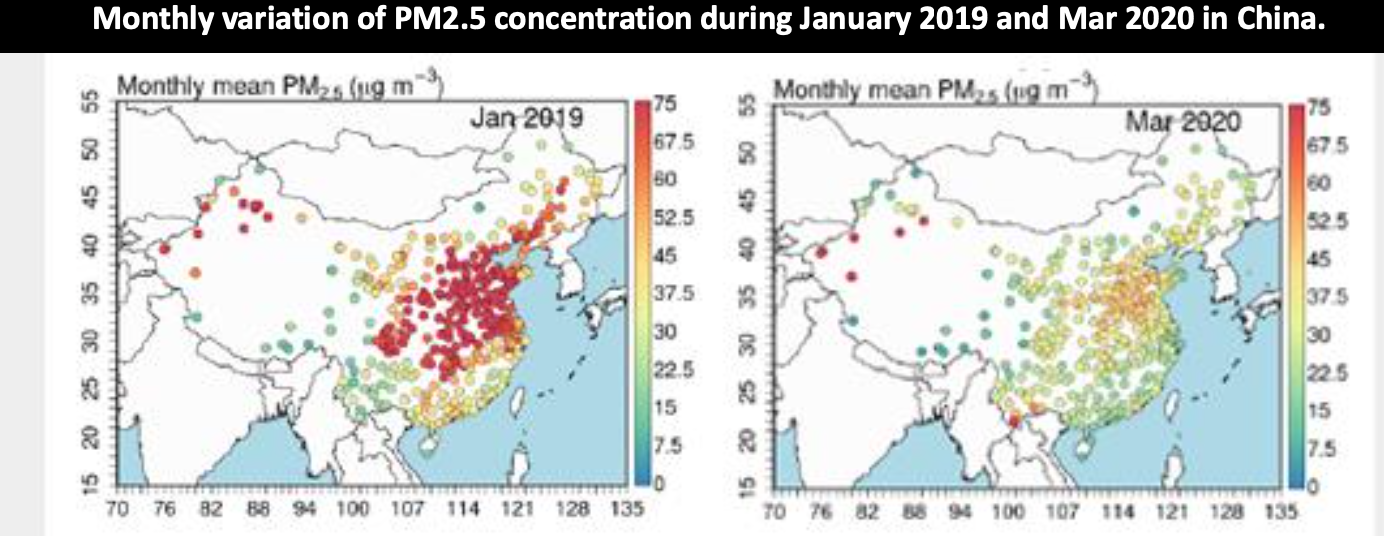
Particulate pollution (and other types) decreased during COVID lockdowns
Carbon Gases
not a pollutant in terms of health directly
Sources:
- Naturally emitted via
- Animal respiration
- Biogeochemical processes (degradation of organic matter)
- Forest fires
- Anthropogenic sources
- Complete combustion of -bearing fuels ()
- Atmospheric residence time: ~100-200 years
Impacts:
- Contributes to Climate Change
- Impacts vegetation growth rates
- Increases acidity or rain, surface water, and oceans (‘acid rain’, ‘ocean acidification’)
(carbon monoxide) more immediately deadly
Naturally emitted via
- volcanoes, forest fires, coal seams, lightning, organic matter degradation in suboxic environments
Anthropogenic source
- Incomplete combustion of C-bearing fuels when less O2 is available: 2C + O2 = 2CO
- Atmospheric residence time - a few months (reacts with O2 to form CO2)
- Natural sources >> anthropogenic sources
- Most abundant of all air pollutants!
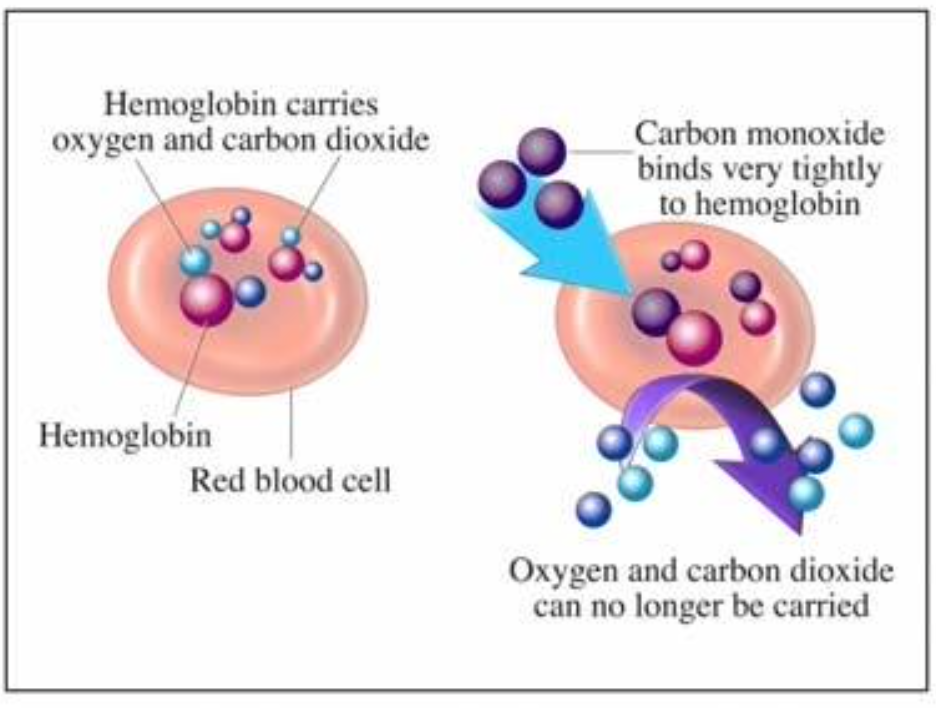
CO is invisible, odourless, colourless, and tasteless.
Replaces and in hemoglobin in the bloodstream.
Cells begin to die from lack of
CO will leave bloodstream with time, but could cause irreversible damage prior
Vehicle emissions (i.e., inefficient gas burning) contributes most to annual CO emissions
More stringent car emission standards brought pollution and deaths down
Accidental death still possible in homes with leaks from gas stoves or furnaces
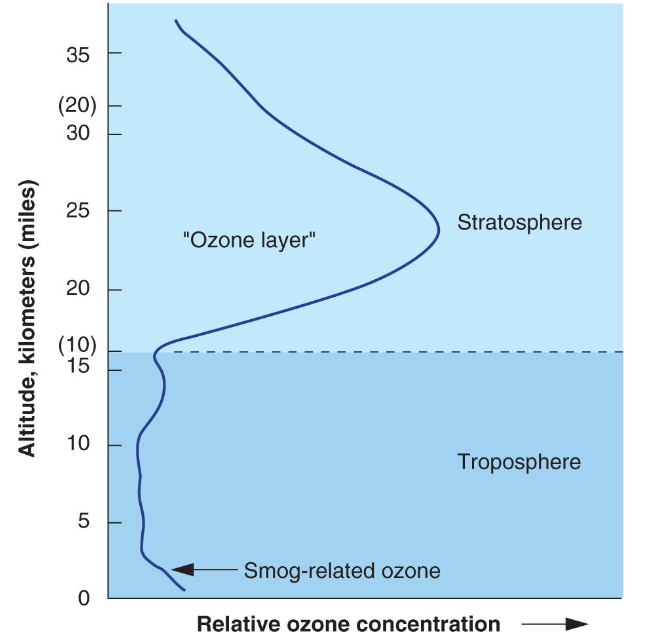
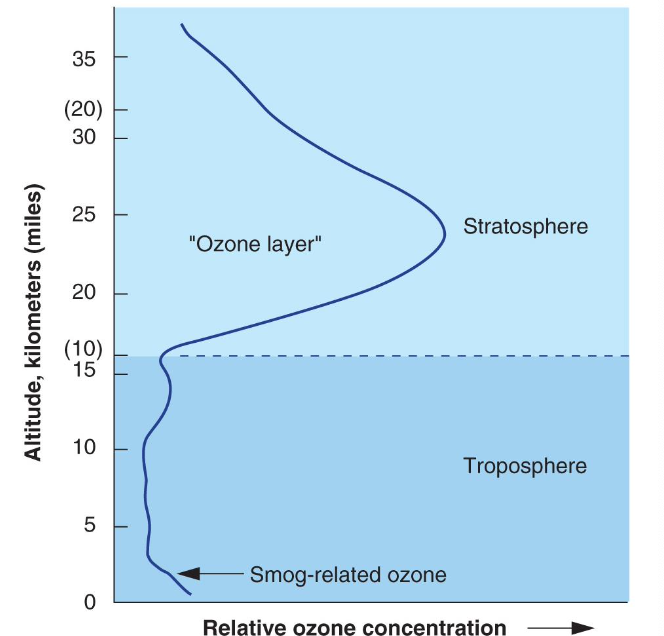
Two Ozone () Layers of Importance in Atmosphere
- in the stratosphere is thee ozone layer that protects us from ultraviolet (UV) radiation
- UV wavelengths divided into UVA (longest and least damaging), UVC (shortest and most damaging), and UVB (most absorbed by ozone and still harmful)
- at ground level (troposphere) is an irritant
- Contributes to smog
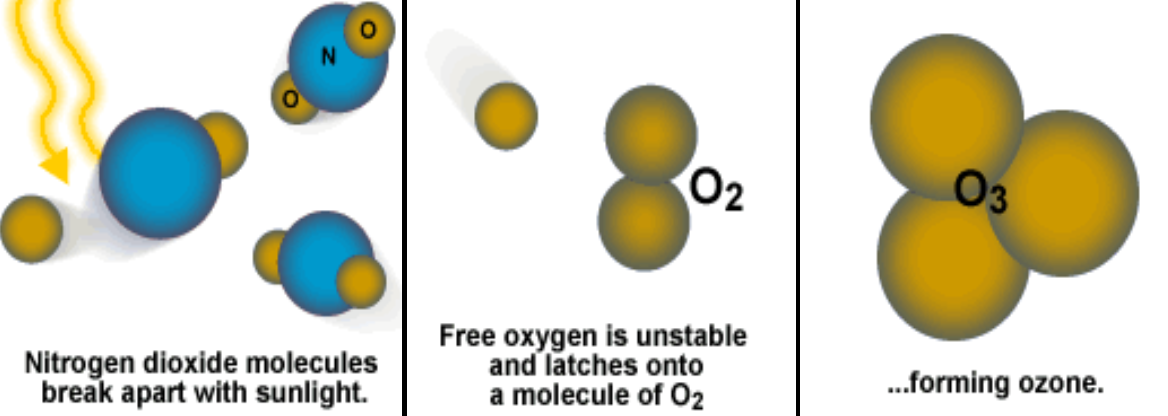
Nitrogen Gases
and are two most abundant elements in the atmosphere, which combines at high temperatures in engines or furnaces to form nitrogen oxides, .
- NO, nitrogen monoxide & , nitrogen dioide
- NO acts like CO in the bloodstream but is not at toxic levels, but will react with to form
- reacts with water vapour and in the atmosphere to form nitric acid (acid rain possible)
- is also a lung irritant and contributes to (ozone) formation in lower atmosphere
Sources:
- Natural sources of includes volcanoes, oceans, lightning, organic matter decay.
- All anthropogenic sources (e.g., engines or furnaces) equivalent to 10% of all natural sources
- Anthropogenic sources concentrated heavily in urban areas
and ‘Smog Ozone’
is an irritant of the airways and lungs, causes asthma and asthma attacks
Sunlight breaks down NO2 and creates ozone, photochemical smog (‘Los Angeles smog’)
- is also a strong lung irritant • Concentrations < 1 ppm can cause harm • also inhibits photosynthesis in plants
Ground ozone occurs mostly in northern hemisphere, where cities and industry are more concentrated
Ozone is swept around the world by westerly winds.
Ozone Layer and Chlorofluorocarbons (CFCs)
- at ground level (troposphere) is an irritant
- in the stratosphere is the ozone layer that protects us from ultraviolet (UV) radiation
- UV wavelengths divided into UVA (longest and least damaging), UVC (shortest and most damaging), and UVB (most absorbed by ozone and still harmful)
and production and destruction all happen in stratosphere
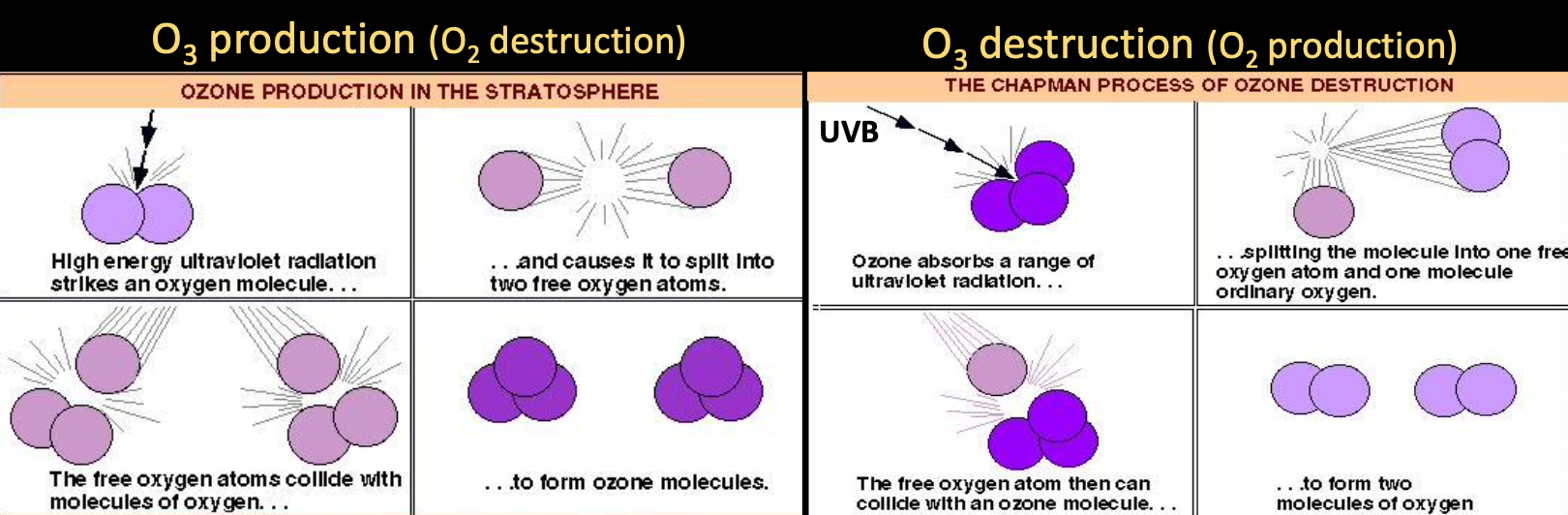
production and destruction is balanced naturally, leaving a layer of ozone that protects us from harmful UVB that causes skin cancer.
- Ozone layer concentrations vary with season and latitude
- Ozone is greatest at high latitudes and UVB exposure is greatest at low latitudes
- But in 1979 a reduction in ozone was noticed over Antarctica ⇒ ozone hole
- Hole peaked in 2006 but has since recovered
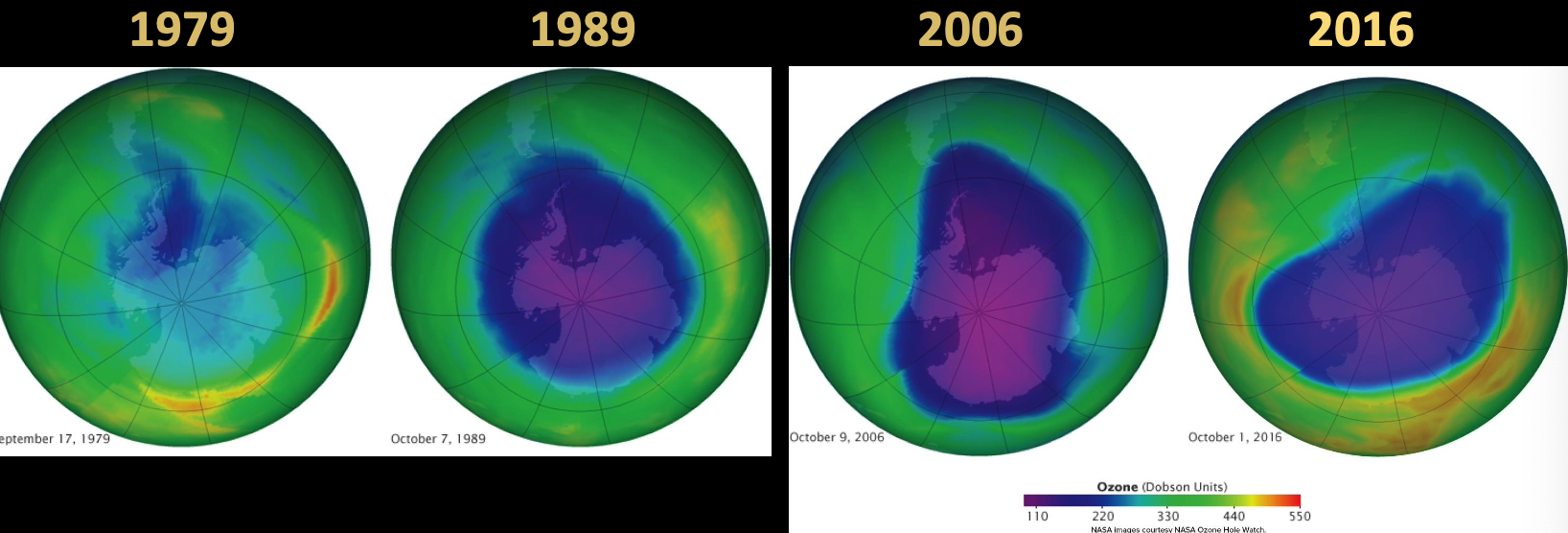
Negative correlation between chlorine monoxide (ClO) and led to studies on the interaction between CFCs and .
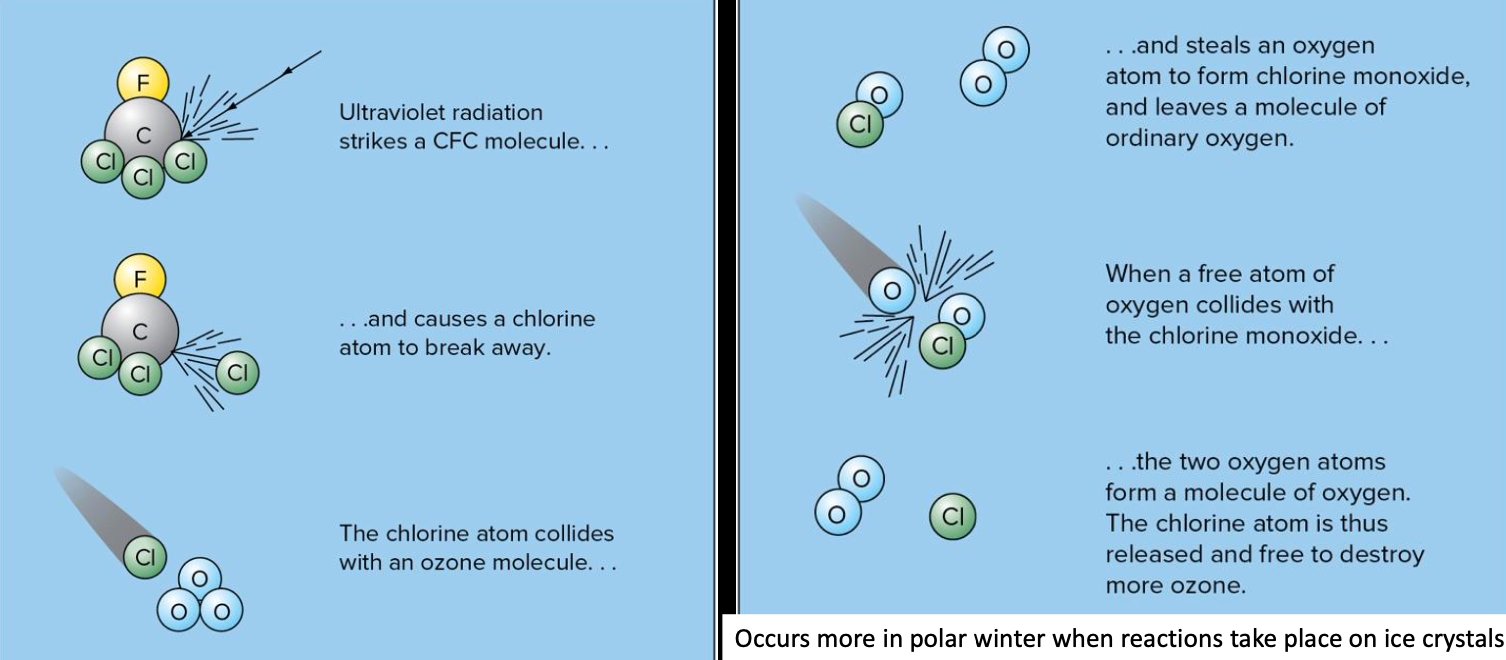
- CFCs were originally created (by American corporations in 1928) to replace toxic chemicals used for refrigeration in refrigerators
- Then they were used in air conditioners, and after WWII as propellants for bug sprays, paints, hair spray, and other health care products
- Most car, house, and business air conditioners were using CFCs by the 1970s, when scientists noticed the correlation between ClO and O3
- Unlike many of the other problems we discussed so far, this was a global one
- 1987 – Montreal Protocol on Substances that Deplete the Ozone Layer – amended and eventually first treaty ever signed by all 197 UN nations guaranteed the banning of CFC use worldwide
- By 2000 all were to be phased out and by 2017 starting to see signs of recovery
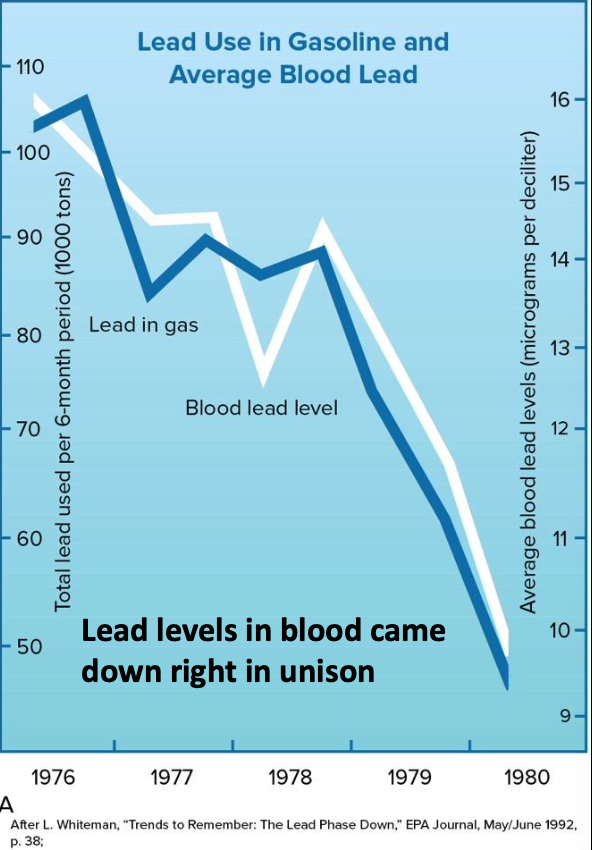
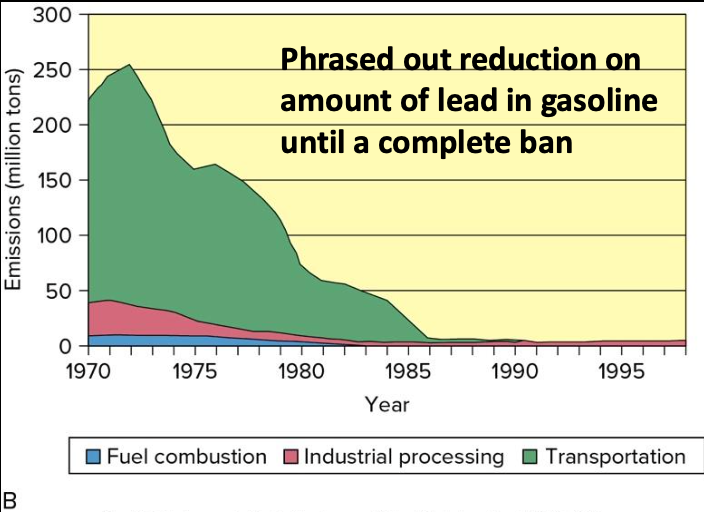
Lead
- Catalytic converters cannot use leaded-fuel and helped improve emissions in other ways
- By 1996 there was a total ban on lead in most vehicles, except in racing and planes
Other Pollutants
- Volatile organic compounds (VOCs) from transportation (unburned gas) and industrial processes
- Not highly toxic but contribute to photochemical smog
- Heavy metals (mercury, lead, cadmium, zinc, arsenic) from minerals-smelters
- As vapour or attached to particulates but quickly removed by precipitation
- Accumulate in local soils, which is a hazard if crops are grown there
- Indoor pollutants
- Radon from housing construction materials or soils near unfinished crawl spaces
- Asbestos from insulation
- Formaldehyde from furniture or carpet
- from fireplace or unvented stove
- VOCs from various cleaners, disinfectants, paints solvents, pesticides (gardening)
Sulfur Gases
- (sulfur dioxide)
- 2/3 from coal combustion in factories and power plants
- Rest from refining and burning petroleum
- Residence time is a few days before reacting with water vapour and : (sulfuric acid)
- In the atmosphere, is irritating to lungs and eyes
- Sulfuric acid is released with precipitation Acid rain
- USA regulations helped reduce emissions by > 75%
Acid Rain - cause by and
- Acidic water readily leaches toxic metals from soils or mining tailings
- Corrodes lots of rock types
- Stunts plant growth
- Causes death or reduction of eggs of creatures near water (birds, fish)

- Easily becomes an international problem because weather travels
- During the 70s and 80s, Canada and USA were at odds over this issue
- 50-70% of the acid rain that falls here and to the east is from US
https://www.bbc.com/future/article/20190823-can-lessons-from-acid-rain-help-stop-climate-change
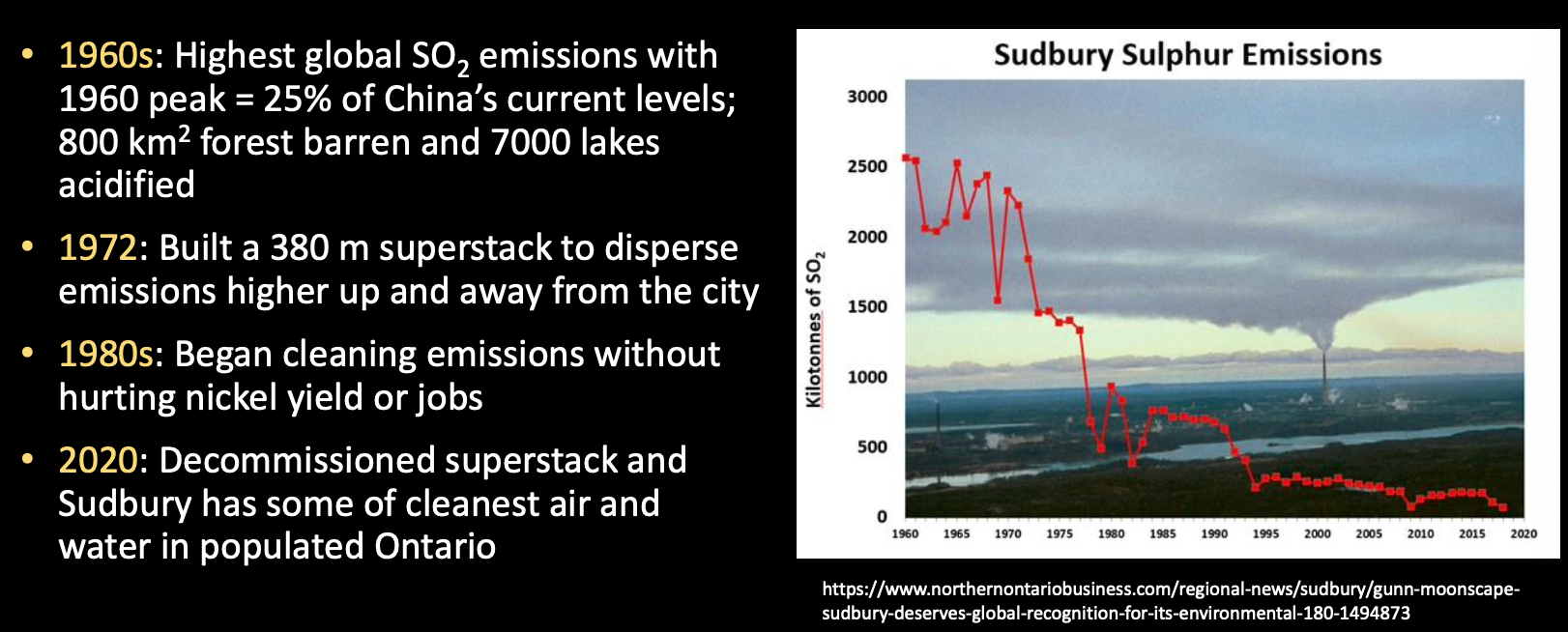
Nickel mining in Sudbury, Ontario went from devastating acid rain story to a rehabilitation success story
Air Pollution and Weather
- Normal: vertical mixing of warm (polluted) air as temps cool with altitude
- Thermal inversion = a layer of cool air occurs beneath a layer of warmer air
- Inversion layer = the band of air in which temperatures rises with altitude
- Denser, cooler air at the bottom of the layer resists mixing
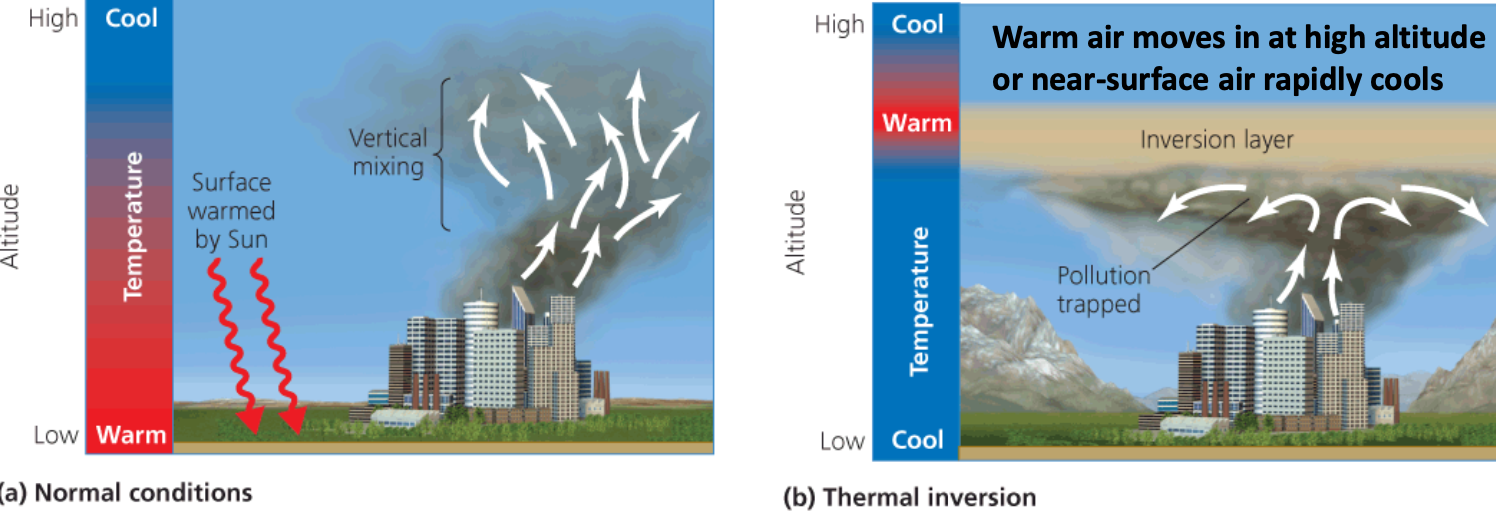
Thermal inversions were the culprit for two of the most famous smog incidences in mid-1900s

- I think the London one was mentioned in the crown…
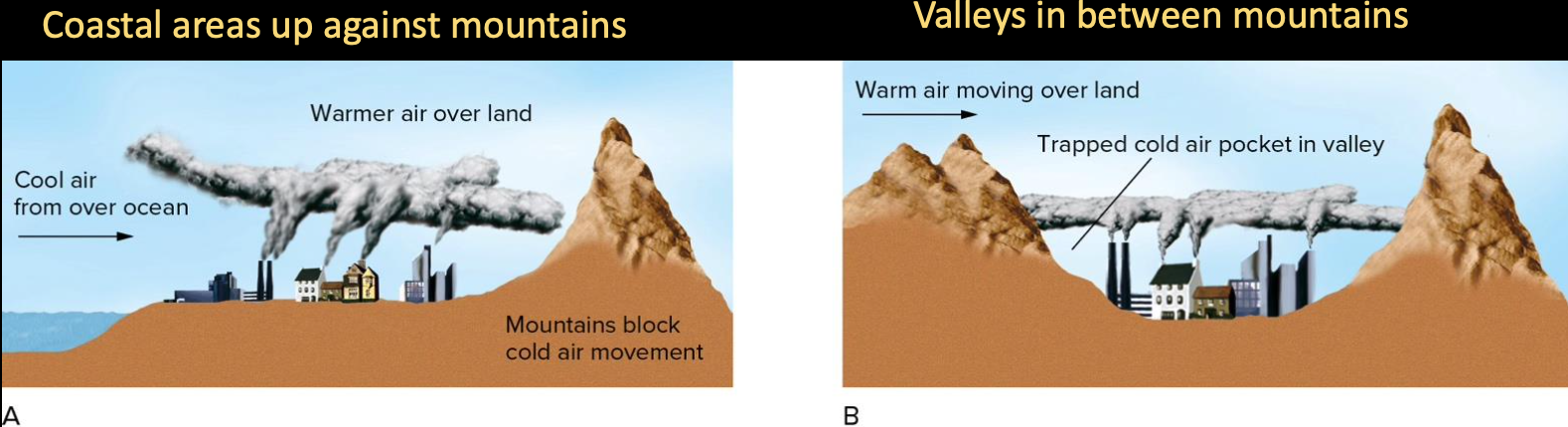
Certain geographic or topographic settings prone to thermal inversions
Particulate pollution can impact weather by modifying temperature through sunlight reflection/absorption that alter winds and by providing surfaces for water vapour to condense.
Toward Air-Pollution Control
- Ozone at ground level
- Particulate Matter ()
- Nitrogen Dioxide ()

Reduce air pollution by trapping it or converting to something less harmful:
- Filters can trap 99.9% of particulates but made from flammable materials
- Electrostatic precipitators to scrub out particulates (98-99.5% effective)
- Costly but used in coal-fire electricity plants
- Wet (chemical) scrubbers pass gas through steam (chemical slurries) to remove particulates and dissolve out some gases
- For sulfur gases in coal-fired plant emissions, use lime () or limestone ()
- Leaves behind contaminated fluids
- CO and emissions can be reduced by lowering combustion temperatures or burning CO to convert to
- Combustion or complex scrubbing destroys organic compounds
- Catalytic converters enhance combustion of HCs to but promote and
- Increase fuel efficiency of cars
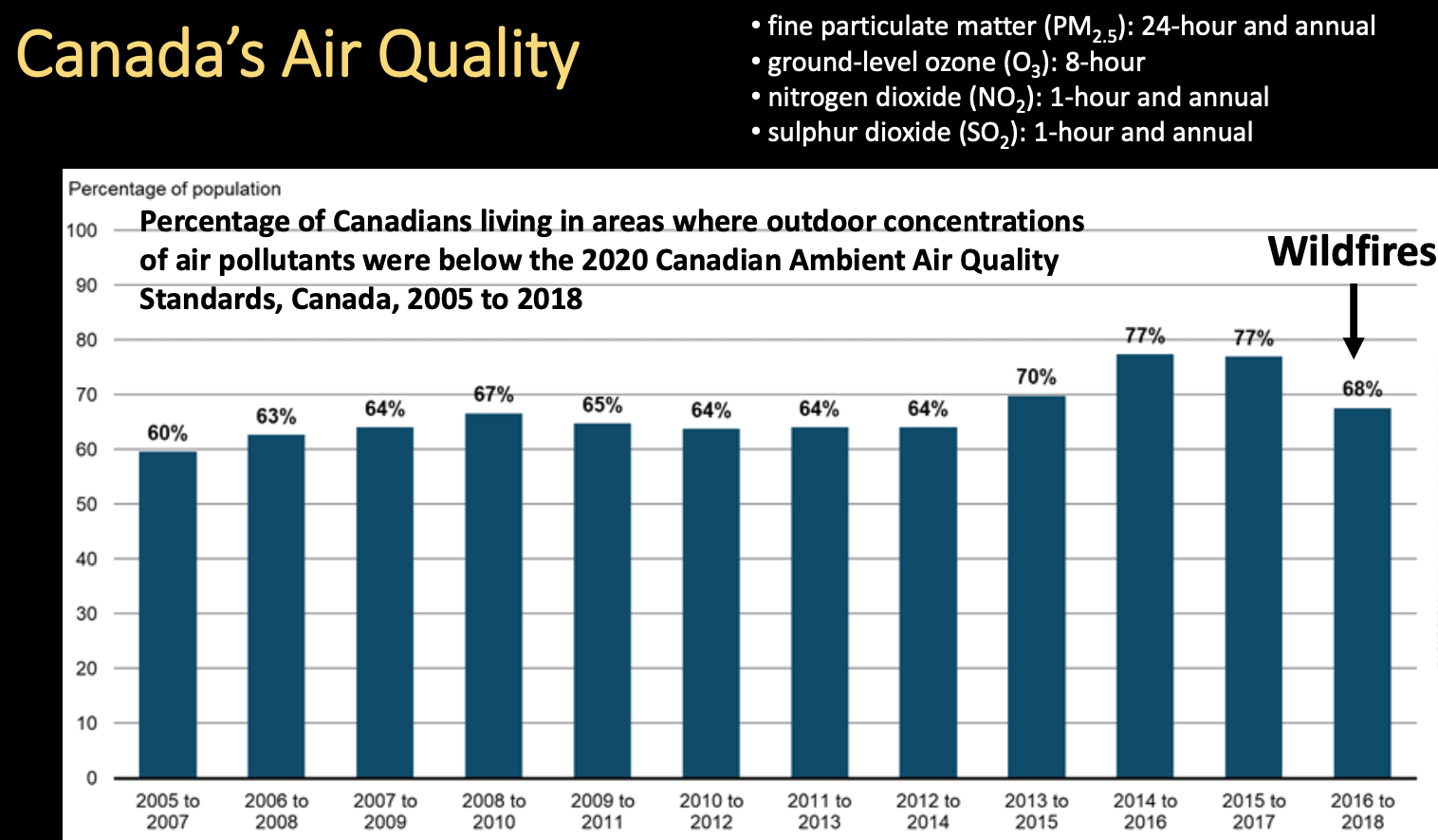
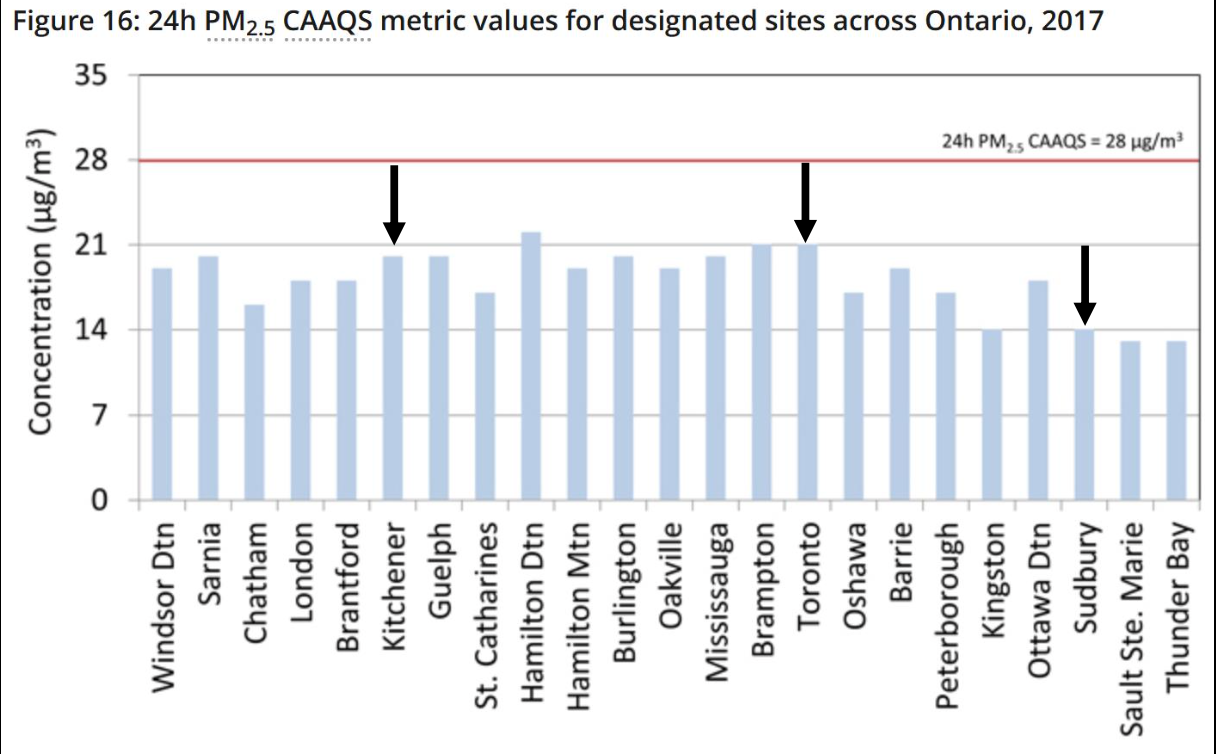
Canada’s Air Quality