Radiometric Dating
Isotopes and Radiometric Dating of Rocks
-
An isotope is an element with different number of neutrons than protons
- Unstable and decays over time into other elements ⇒ radioactive decay
-
Exponential decay you can measure ⇒ half-life
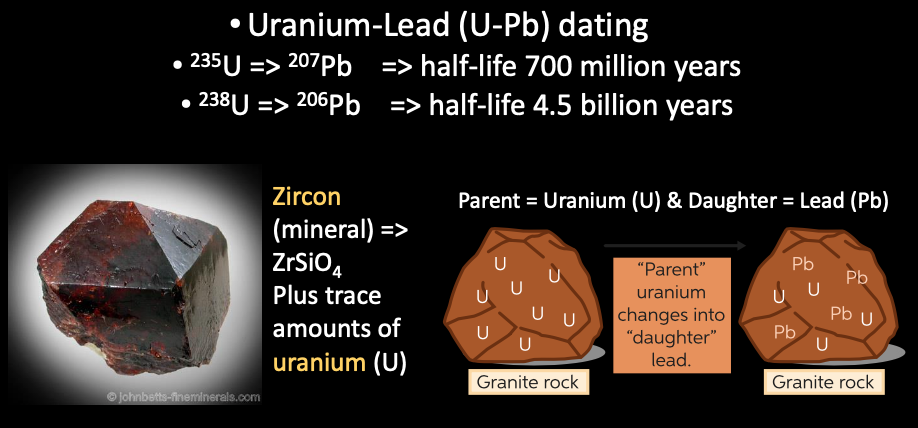
- Radiometric dating ⇒ determine age of rock by back-calculating to original amount
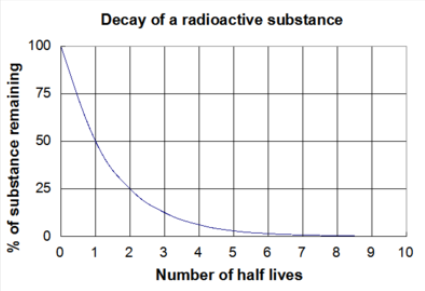
- Radiometric dating ⇒ determine age of rock by back-calculating to original amount
-
Carbon has 3 isotopes ⇒
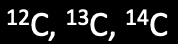
- 12C has 6 protons, 6 neutrons; 13C has 6 protons, 7 neutrons; 14C has 6 protons, 8 neutrons
- 14C decays over time to 14N
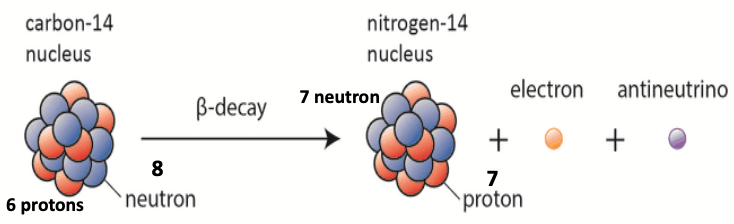
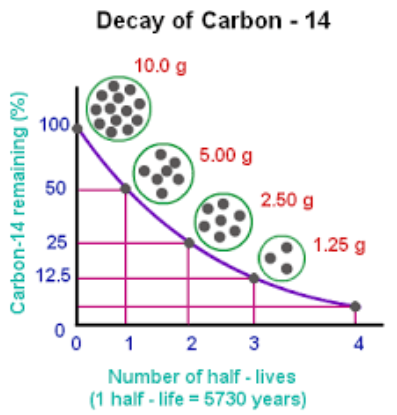
- 14C Half-life = ~5700 years
- Only date things ~50,000 years old
→ Ion